Watts, R. J.; Harrington, J. S.; Van Houten, J. J. Am. Chem. Soc. 1977, 99, 2179.
Wickramasinghe, W. A.; Bird, P. H.; Serpone, N. J. Chem. Soc., Chem Commun. 1981, 1284.
In terms of the application importance and the research extent, perhaps the iridium counterpart of Ru(bipy)3 is Ir(ppy)3,
where ppy stands for 2-phenylpyridine, [CAS 1008-89-5] . It is also interesting to compare the bis-ligand metal chloride of
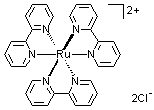
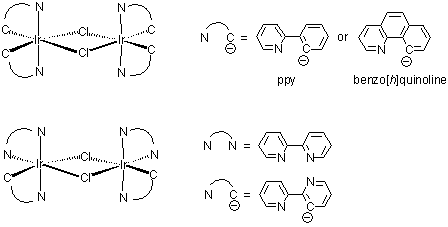
ruthenium and iridium. For ruthenium, a usual compound is cis-Ru(bipy)2Cl2, a mononuclear species; while in the case of
iridium, the counterpart is a dichloro-bridged dimer, i.e., dichlorotetrakis(2-(2-pyridinyl)phenyl)diiridium(III), [92220-65-0].
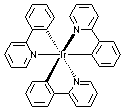
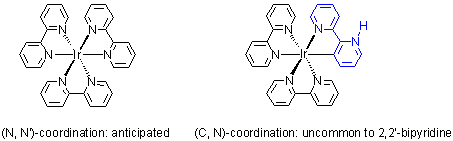
Even with 2,2'-bipyridine, iridium still can form dichloro-bridged dimer with one (N, N')-coordinated bipy and one (N, C)-
coordinated bipy (see below structures). Some dichloro-bridged dimers are available from Rubipy Scientific Inc. (see the
following table)
Between ruthenium (N, N')-coordinated compounds and iridium (C, N)-cyclometallated compounds, difference exists also in
the tunability of emission wavelengths. While most of the Ru (N, N')-coordinated compounds have luminescent emission
narrowly around 610 nm, Ir (C, N)-cyclometallated compounds can emit light from blue to infrared. The broad tunability plus
other properties make Ir complexes very useful in the OLED display and lighting technology.
Unfortunately, the syntheses and purification of tris-cyclometalated iridium complexes are fairly troublesome, leading to
yields generally much lower than those of most ruthenium (N, N')-coordinated compounds.
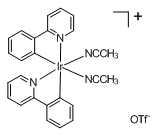
Bis-cyclometallated Ir(III) compounds which contain labile solvents(DMF, DMSO, MeCH and water etc.) as ligands may find
uses in a number of synthetic and analytical processes. RSI is now marketing one of such complexes, bis(2-phenylpyridine-
C2,N’)(acetonitrile)iridium(III) trifluoromethanesulfonate (Cat# IR601601MECN) for bioanalytical application. Because the
two solvent molecules (MeCN) can be readily replaced by histidine. This complexes can be used to detect histidine-rich
proteins or polypeptides.
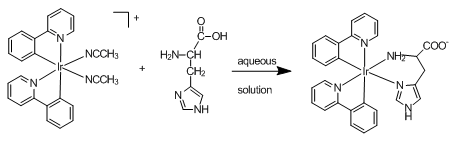
The product of the reaction between histidine and bis(2-phenylpyridine-C2,N’)
(acetonitrile)iridium(III) trifluoromethanesulfonate is stable and probably has the
structure showing in the following scheme.
Cat# IR601601MECN
Schmid, B.; Garces, F.O.; Watts, R.J.
Inorg. Chem. 1994, 33, 9-14